LifeBridge Innovations story began with the effort to save one life and the discovery to extend the life of thousands.
Laurie M. Travers had struggled with stage 4 breast cancer through nine reoccurrences over a period of 15 years. By late 2012, she had developed severe cancer in the pleura around the lung. The fluid buildup in the pleura was compressing her left lung, making it difficult to breathe. Every three weeks, Laurie had fluid withdrawals from the pleura (Thoracentesis) of between 900 and 1,500 cc. Laurie’s cancer had become reoccurring and non-responsive to most treatments. Laurie and her family thought the end was imminent.
Click on image to view larger
During this time, Laurie's husband Peter heard of the new Tumor Treating Fields therapy approved by the FDA for Glioblastoma. He quickly learned it would not be available for people like Laurie for 10 or more years. Determined to help his wife, Peter formed a team of friends and family, including a senior engineer he met through church. After intensive work for hundreds of team hours, a new TTF device meeting the standards of present research was completed.
https://www.ted.com/talks/bill_doyle_treating_cancer_with_electric_fields
Shortly thereafter, Laurie had her sixth Thoracenteses, which produced 900 cc of fluid. The next day, Laurie began wearing the new TTF device at least 12 hours per day while continuing her chemotherapy. Three weeks later, her seventh Thoracenteses produced only 500+ cc; the eighth, 240cc; and the ninth only negligible fluid was found. The cancer in the pleura had been resolved. During this entire time, Laurie’s tumor markers increased, indicating the cancer was spreading, which did not make sense in light of the improvement. More scans were ordered.
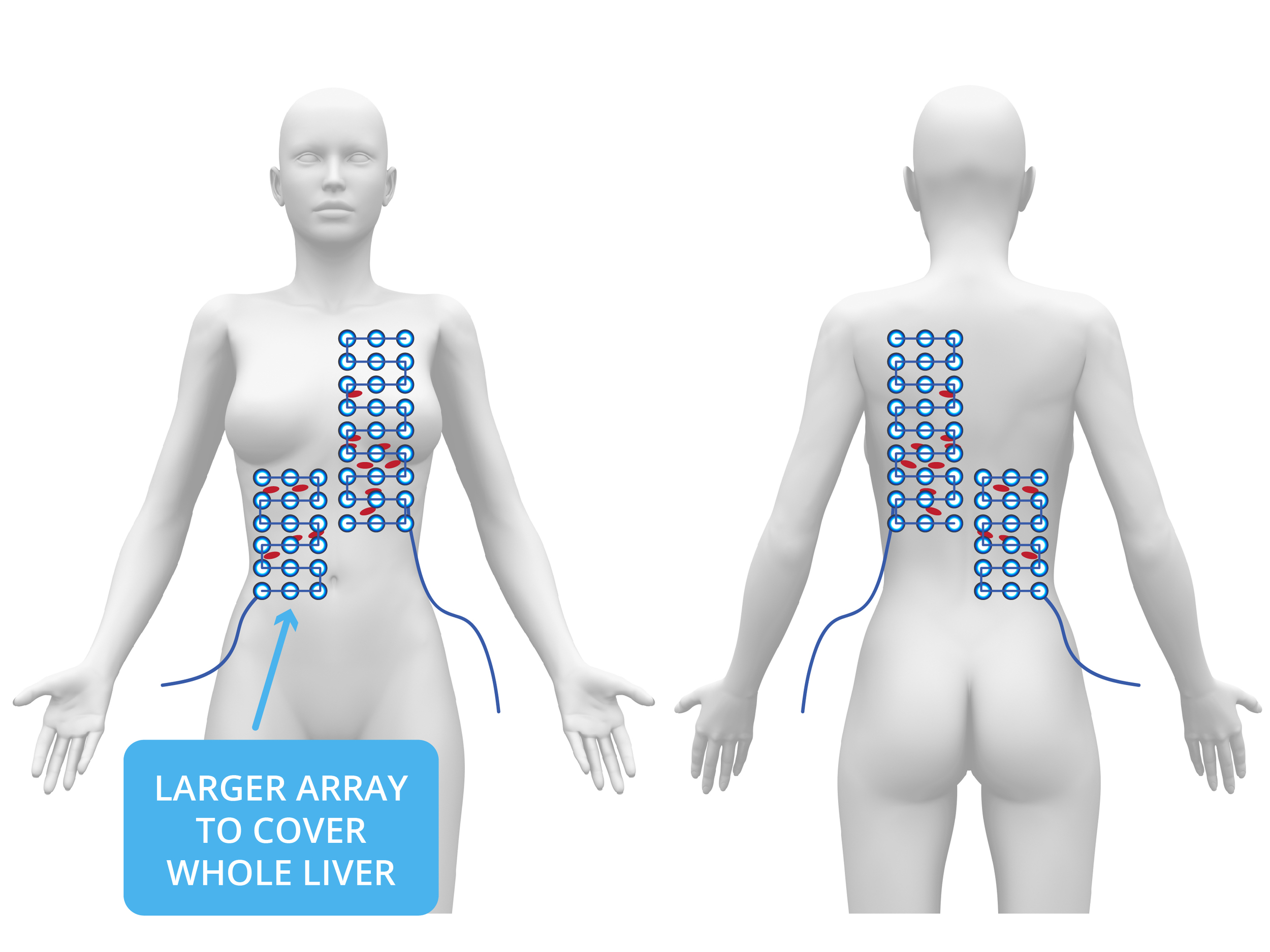
The scans revealed that new cancer had broken out on the top of the liver. The chemotherapy was called a failure and a new one was started. The team immediately produced a second TTF array over the top of the liver and Laurie continued wearing the TTF 12 hours per day. The TTF had no side effects other than very minor skin irritations. Laurie returned to work as a neonatal nurse in between chemo cycles and taught a young women's bible study at church.
Over the next months, the tumor markers continued to increase, so additional scans were ordered. These scans showed continued stability in the pleura and a reduction in the tumors at the top of the liver where TTF had been applied. However, new tumors were now growing in the lower section of the liver, outside the range of the TTFs. The second chemo was also declared a failure. The liver TTF array was extended downward to cover the entire liver and a third chemo was started.
Laurie’s quality of life remained high as the third chemo was attempted. The same pattern continued and after several months, the higher tumor markers required additional scans. This time, the results showed a game-changing situation.
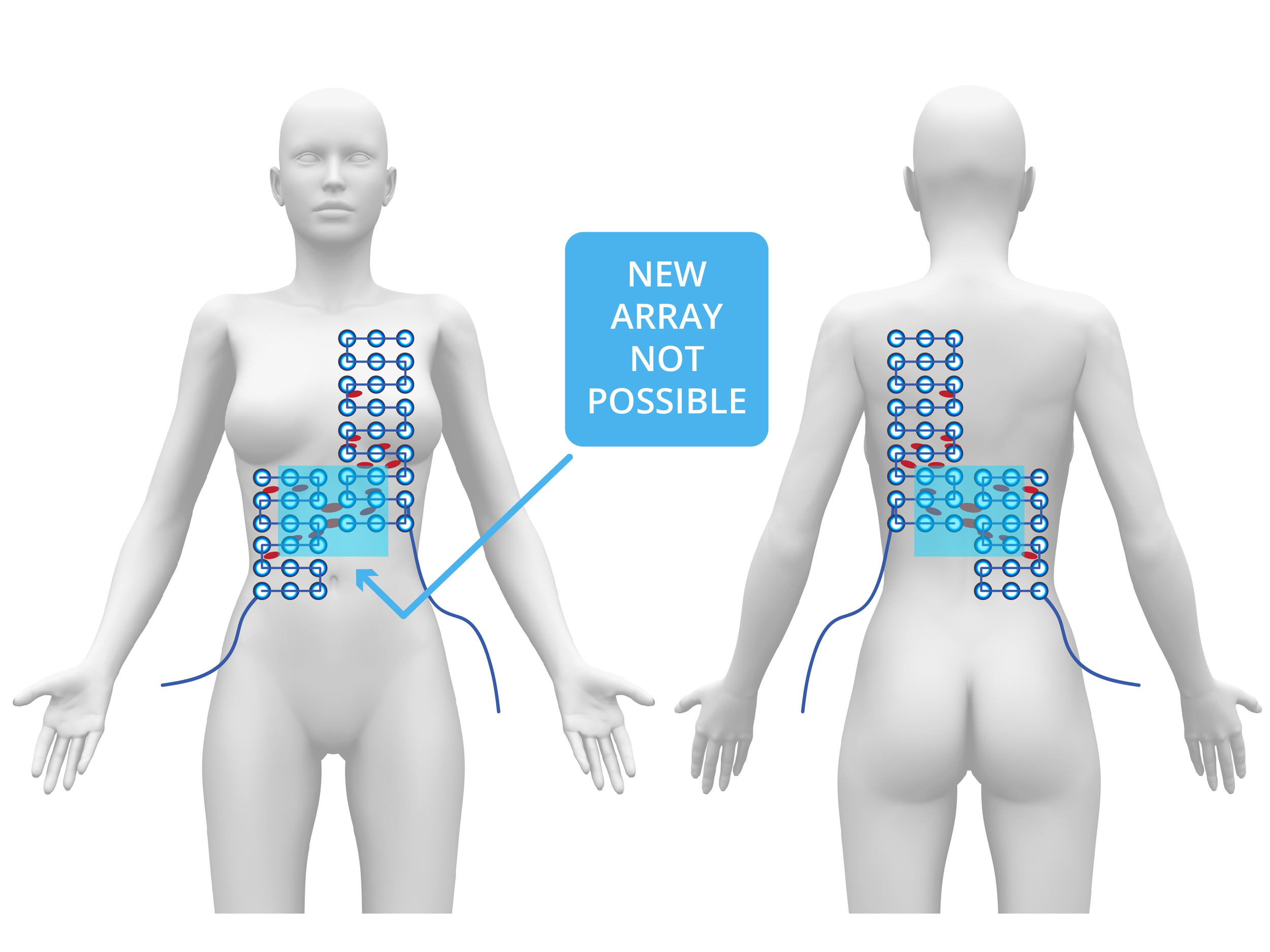
Cancer reduction and stability in the liver and pleura were noted, but new growth had broken out in the peritoneal cavity between the liver and lung TTF arrays. For the first time, we had reached the limits of static TTF therapy. A new array was needed over the peritoneal cavity, but was not possible because arrays cannot be placed on top of other arrays. TTFs can be too weak to kill cancer around the edges of arrays—central targeting is needed to maximize killing power.
This left Peter and his team with the excruciating decision of which cancer to treat and which to let grow. This was the beginning of the end for Laurie. For the next 12 to 18 months, wherever the TTF was applied, stability or reduction of the cancer occurred, while the spread of the disease continued outside the range of the TTF’s.
With two senior engineers on the team, Peter’s group began inventing a better way to deliver TTF. During the new system development, Laurie began taking a promising chemotherapy that was beginning to show tumor marker reductions. She elected to only take the heavy chemotherapy and forgo wearing the partially developed new version of TTF. Within two-and-a-half months, she could no longer tolerate the chemotherapy and began missing doses. Laurie passed away surrounded by family in May of 2016.
Laurie lived an additional three years from the time of severe cancer in the pleura. One year in, she had a “Sweet 16” celebration, giving thanks for 16 years of cancer survival. Laurie expressed her feelings in a note to friends.
“Peter & I have spent the whole last week just in awe of God’s goodness & the love of friends. The joy of the celebration has lingered in our (heart emoji)’s. Considering how desperate life looked with each drain of my lung last year at this time - God’s miraculous turn-around is awesome. I feel so blessed to have been given more life to live -”
Inspired by Laurie’s life, the team at LifeBridge Innovations continues researching and developing its patented LB10000 device, for future FDA approval. The device is being developed from the ground up for late-stage cancer patients with metastatic disease in two or more locations on the body at once. No longer will those treating metastatic patients with TTF be left with the painful decision of what cancer to treat and which to let grow.